Abstract
Background
Complications of sickle cell disease (SCD) include pain episodes, chronic anemia, cardiopulmonary disease, and long-term end-organ damage. Hydroxyurea is efficacious in preventing these complications by increasing fetal hemoglobin levels (HbF); however, adherence is limited. Metformin, a Food and Drug Administration-approved drug used for type-2 diabetes mellitus (DM), was recently found to induce HbF through upregulation of FOXO3 and gamma-globin expression. However, the relation of metformin utilization to clinical outcomes and healthcare utilization in adults with SCD and DM has not been studied.
Objectives
Our specific aims were to 1) estimate the prevalence of DM in a cohort of adult patients with SCD and 2) assess the association of metformin with clinical outcomes and healthcare utilization in this population. We hypothesized that adults with SCD and DM on metformin have less frequent SCD-related complication and lower utilization of hospital care for those complications.
Methods
We analyzed subjects with SCD and DM ≥18 years of age from the Truven Health MarketScan® Multi-State Medicaid Database from 2009-2015 with continuous enrollment of ≥365 days. Subjects with SCD and DM were identified through the presence of ≥1 inpatient or ≥2 outpatient disease-specific billing codes or ≥1 outpatient DM code and ≥1 DM-related drug claim. Subjects with drug claims for insulin, hydroxyurea, or iron chelation were excluded. Metformin use was defined as having ≥1 metformin-related drug claim in the study period. Outcomes included annual rates of all-cause inpatient hospitalizations, SCD-related hospitalizations, all-cause emergency department (ED) visits, SCD-related ED visits, vaso-occlusive episodes (VOE), strokes, acute chest syndrome (ACS) episodes, avascular necrosis (AVN) events, and gallstone events. Outcomes were identified based on claims records after the subject's first DM diagnosis record in the dataset. Clinical events among metformin users and non-users were compared using chi square tests. Rate ratios (RR) comparing annual rates of clinical events among metformin users to non-users were estimated using negative binomial regression, controlling for age, sex, and claims-data-defined Charlson Comorbidity Index (CCI) score.
Results
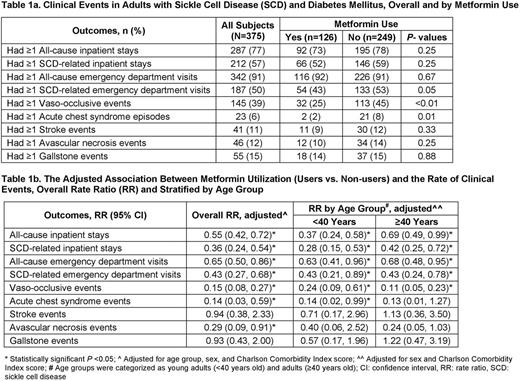
We identified 7,617 subjects with SCD and 1,296 (17%) of them also had a diagnosis of DM. Our final cohort included a total of 375 subjects who met all our pre-defined criteria; of them, 126 (34%) were treated with metformin. Subjects were 72% female and 82% Black, and had a median age of 44 years (interquartile range (IQR) 33-52) and CCI score of 2 (IQR 0-4). All clinical events were less frequent in metformin users when compared to non-users, summarized in Table 1a, and subjects in both groups were similar in age, sex, race and CCI score. Using negative binomial regression, adjusted for age, sex and CCI score, metformin users had significantly lower all-cause inpatient stays (rate ratio (RR) 0.55; 95% confidence interval (CI) 0.42-0.72), SCD-related inpatient stays (RR 0.36; 95% CI 0.24-0.54), all-cause ED visits (RR 0.65; 95% CI 0.50-0.86), SCD-related ED visits (RR 0.43; 95% CI 0.27-0.68), VOE (RR 0.15; 95% CI 0.08-0.27), ACS (RR 0.14; 95% CI 0.03-0.59) and AVN (RR 0.29; 95% CI 0.09-0.91), compared to metformin non-users (Table 1b). Similar trends were also seen when patients were stratified by age as young adults (<40 years) vs. adults (≥40 years), as shown in Table 1b.
Conclusions
This study is the first to examine clinical associations of metformin use with SDC-related complications in adults with SCD and DM. These findings strongly suggest that metformin has important protective effects on all-cause and SCD-related hospital-treated clinical events. Among adults with SCD and DM, metformin users had 45% fewer SCD-related inpatient stays and roughly 85% fewer VOE or ACS episodes after controlling for confounding variables. These benefits are consistent with the effects of metformin on HbF induction observed in earlier laboratory data. An analysis of the effects of adherence to metformin on clinical outcomes and healthcare utilization in our cohort is ongoing. Given our findings and the known safety profile of metformin, future prospective studies are warranted to evaluate its efficacy and cost-effectiveness in relation to clinical outcomes, quality of life, mortality and healthcare utilization in adults with SCD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal